what mass of iron must react to produce 3600 kj of energy
Affiliate 7. Energy and Chemistry
Stoichiometry Calculations Using Enthalpy
- Perform stoichiometry calculations using energy changes from thermochemical equations.
In Chapter 5 "Stoichiometry and the Mole", we related quantities of one substance to another in a chemic equation past performing calculations that used the balanced chemical equation; the counterbalanced chemical equation provided equivalences that we used to construct conversion factors. For case, find the following balanced chemical equation:
![]()
In this equation, we recognize the following equivalences:
![]()
Where ⇔ is the mathematical symbol for "is equivalent to." In our thermochemical equation, all the same, we take some other quantity — energy alter:
![]()
This new quantity allows u.s. to add some other equivalence to our list:
![]()
That is, nosotros tin can now add an energy amount to the equivalences — the enthalpy modify of a balanced chemical reaction. This equivalence tin as well be used to construct conversion factors so that we tin can relate enthalpy change to amounts of substances reacted or produced.
Annotation that these equivalences address a business organisation. When an amount of free energy is listed for a balanced chemical reaction, what amount(s) of reactants or products does it refer to? The answer is that relates to the number of moles of the substance equally indicated by its coefficient in the balanced chemical reaction. Thus, ii mol of H2 are related to −570 kJ, while ane mol of O2 is related to −570 kJ. This is why the unit of measurement on the energy change is kJ, not kJ/mol.
For instance, consider the following thermochemical equation:
![]()
The equivalences for this thermochemical equation are:
![]()
Suppose we asked how much energy is given off when 8.22 mol of Htwo react. We would construct a conversion gene betwixt the number of moles of H2 and the energy given off, −184.6 kJ:
![]()
The negative sign means that this much free energy is given off.
Problem
Determine how much free energy is given off when 222.4 grand of North2 reacts in the following thermochemical equation:
![]()
Solution
The counterbalanced thermochemical equation relates the energy modify to moles, not grams, then nosotros first convert the amount of N2 to moles and then use the thermochemical equation to determine the energy change:
![]()
Examination Yourself
Determine how much oestrus is given off when one.00 g of Hii reacts in the following thermochemical equation:
![]()
Respond
−xv.i kJ
Like whatever stoichiometric quantity, we can start with energy and determine an amount, rather than the other fashion around.
Problem
Determine what mass of NO can be made if 558 kJ of free energy are supplied, given the following thermochemical equation:
![]()
Solution
This time, we kickoff with an amount of free energy:
![]()
Exam Yourself
Given the following equation, how many grams of N2 will react if 100.0 kJ of energy are supplied?
![]()
Reply
15.5 k
Chemistry Is Everywhere: Welding with Chemical Reactions
One very energetic reaction is called the thermite reaction. Its classic reactants are aluminum metallic and iron(III) oxide; the reaction produces iron metallic and aluminum oxide:
![]()
When properly done, the reaction gives off so much energy that the iron product comes off equally a liquid. (Iron usually melts at 1,536°C.) If carefully directed, the liquid iron can fill spaces between ii or more than metal parts and, after it quickly cools, can weld the metallic parts together.
Thermite reactions are used for this purpose fifty-fifty today. For civilian purposes, they are used to re-weld broken locomotive axles that cannot be easily removed for repair. They are used to weld railroad tracks together. Thermite reactions tin can also be used to separate thin pieces of metal if, for whatever reason, a torch doesn't work.

Thermite reactions are also used for military purposes. Thermite mixtures are oftentimes used with additional components as incendiary devices — devices that start fires. Thermite reactions are also useful in disabling enemy weapons: a slice of arms doesn't work so well when information technology has a hole melted into its barrel because of a thermite reaction!
- The free energy change of a chemical reaction can exist used in stoichiometry calculations.
Questions
- Write the equivalences that this balanced thermochemical equation implies:
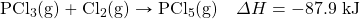
- Write the equivalences that this balanced thermochemical equation implies:
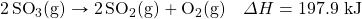
- How many kilojoules are given off when 17.eight mol of CHfour(yard) react in the post-obit equation?
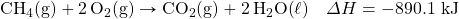
- How many kilojoules are captivated when 0.772 mol of N2(grand) reacts in the post-obit equation?
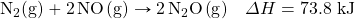
- How many kilojoules are absorbed when 23.09 mol of CsixHhalf-dozen(ℓ) are formed in the following equation?
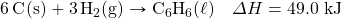
- How many kilojoules are given off when 8.32 mol of Mg react in the following equation?
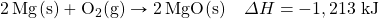
- Glucose (C6H12O6) is the main fuel metabolized in animal cells. How much energy is given off when 100.0 g of C6H12O6 react in the following equation?
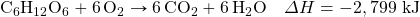
- How much energy is given off when 288 k of Fe are produced, given the post-obit thermochemical equation?
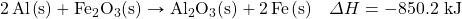
- How much energy is absorbed when 85.two g of CO2 are reacted in the following thermochemical equation?
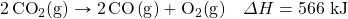
- How much energy is absorbed when 55.nine thousand of Na+(aq) are reacted in the following thermochemical equation?
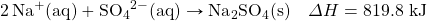
- NaHCO3 decomposes when exposed to heat. What mass of NaHCOiii is decomposed by 256 kJ, in the following thermochemical equation?
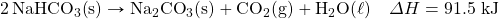
- HgO decomposes when exposed to heat. What mass of O2 tin be made with 100.0 kJ, in the post-obit thermochemical equation?
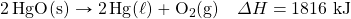
- What mass of And so3 is needed to generate 1,566 kJ in the following thermochemical equation?
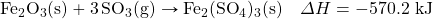
- What mass of HBr volition exist formed when 553 kJ of energy are given off in the following thermochemical equation?
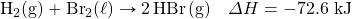
Answers
- 1 mol PCl3 ⇔ 1 mol Clii ⇔ 1 mol PClfive ⇔ −87.9 kJ
- 15,800 kJ
- 1,130 kJ
- 1,554 kJ
- 548 kJ
- 470 grand
- vi.60 × 10two g
Media Attributions
- "Thermite Reaction" by Skatebiker © Public Domain
greenhalghlaregrell.blogspot.com
Source: https://opentextbc.ca/introductorychemistry/chapter/stoichiometry-calculations-using-enthalpy/
0 Response to "what mass of iron must react to produce 3600 kj of energy"
Post a Comment